Kenneth Fox
With the Pfizer/BioNTech, Moderna and Astrazenca vaccines all being approved by the European Medicines Agency (EMA), the next vaccine in line for approval is the Johnson & Johnson vaccine.
To date, Ireland has administered 180,192 first doses of the vaccine, while 91,750 second doses have been given out.
There is a lot of hope that the J&J vaccine - made by its subsidiary Janssen - will be able to help accelerate the vaccine rollout because it is easier to administer. It is not only easier to store it also requires only one dose.
How effective is the Johnson & Johnson vaccine?
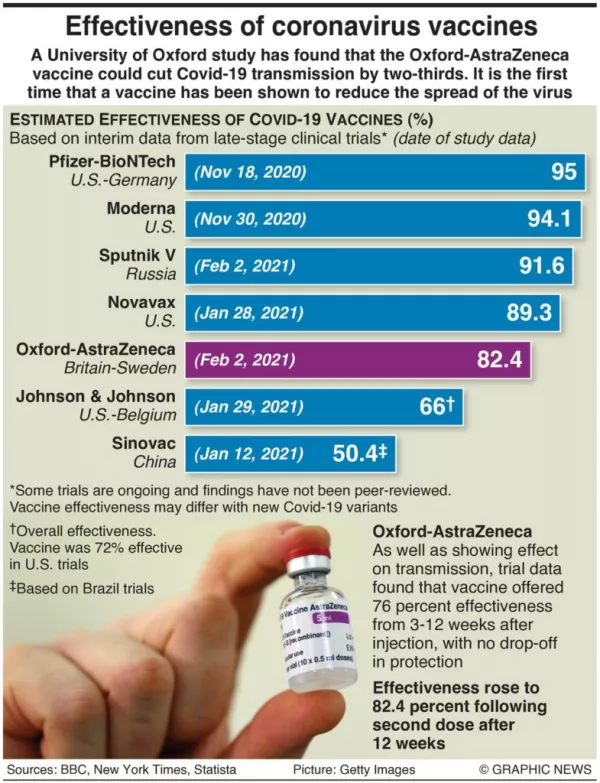
The vaccine is 66 per cent effective at preventing moderate to severe Covid-19 but offers high protection against people needing to go to hospital.
The company claims that in terms of prevent severe disease, its jab was 85 per cent effective “and demonstrated complete protection against Covid-19 related hospitalisation and death as of day 28”.
What makes it different to the vaccines we already have?
With the vaccine being one dose, it can be rolled out across populations much more quickly than two-dose vaccines.
Another major advantage is that it does not require ultra-cold storage like the jabs from Moderna and Pfizer. It can be kept at fridge temperature for at least three months.
This is compared to the Pfizer vaccine which has to be kept in a freezer at -70 degrees and the Moderna vaccine which has to be kept at -20 degrees. The Oxford-AstraZeneca vaccine meanwhile only needs to be stored at 2-8 degrees.
How exactly does the J&J vaccine work?
Unlike the mRNA vaccines from Moderna and Pfizer, the J&J vaccine uses an adenovirus – which is a type of virus that causes the common cold which has been modified - so it cannot replicate and cause illness.
This is very similar to the approach used by Oxford University and AstraZeneca, which have also used an adenovirus.
We already know that the Covid-19 virus is studded with spike proteins that it uses to enter human cells – these spike proteins are the target for vaccines.
The J&J vaccine is based on the virus’s genetic instructions for building the spike protein and uses double-stranded DNA.
The gene for the coronavirus spike protein has been added to the adenovirus. Following injection, this genetic material is read by the body’s cells to mount an immune response to the perceived threat.
If, later on, the vaccinated person comes into contact with the virus, the immune system is prepared to attack it.
Has this technology been used before?
J&J has used this technology before in an Ebola vaccine and in so-called 'investigational' vaccines for HIV, both of which have accrued long-term safety data.
Experts believe the immune system contains special cells called memory B cells and memory T cells, which might retain information about the coronavirus for a long time.
When can we expect it to be available for use in Ireland?
As The Irish Times reports, the EMA started a rolling review of this single dose vaccine from Janssen, the Belgium-based subsidiary of Johnson & Johnson on December 1st.
The EU has agreed an initial contract for 200 million doses, with an option to buy 200 million more, assuming it secures regulatory approval.
On February 16th, the company applied to the EMA for seeking authorisation for its single-dose vaccine, paving the way for its availability in Ireland by April. - (Additional reporting from PA)












